April 22, 2023 : to advance research
Meet in Strasbourg for the Silent Line
5 April 2023In 2021, in France, 1,893,897 million animals were sacrificed for scientific purposes.
According to The Lancet, based on a memo from the European Commission, 197,000 Europeans die prematurely each year as a result of the side effects of drugs that have however been extensively tested on animals.
The rate of failure when switching from animals to humans oscillates between 80 and 99%
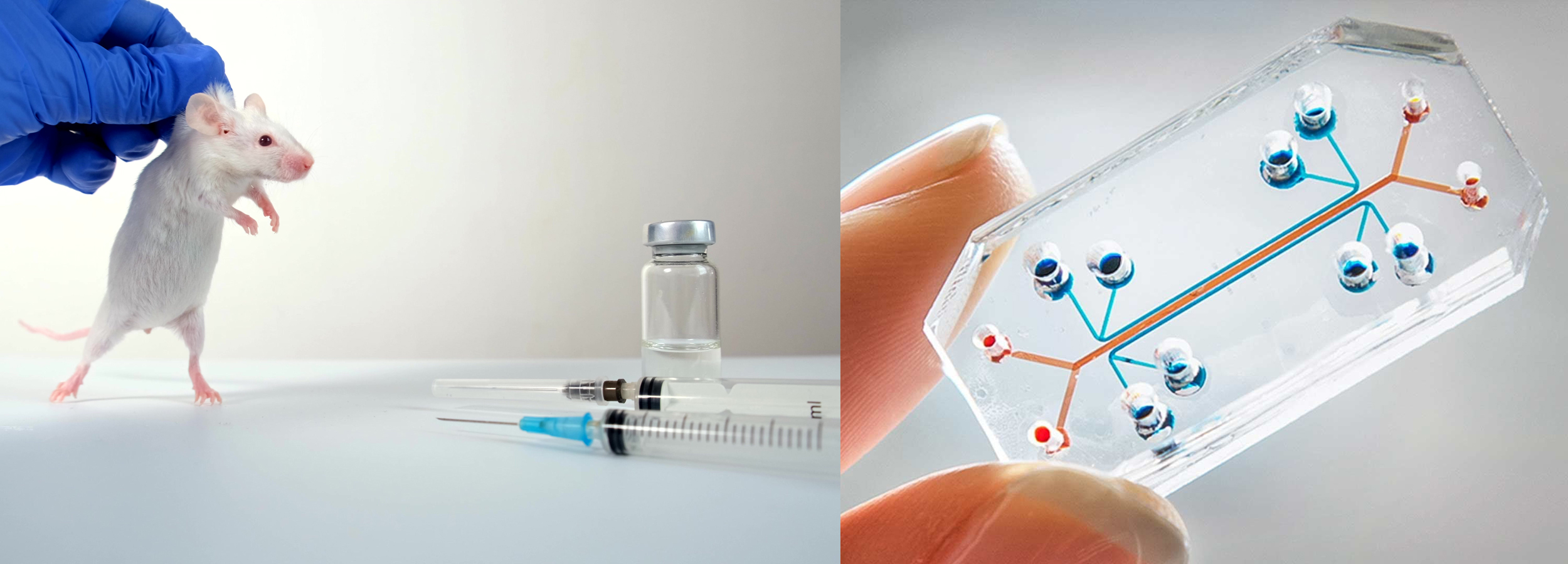
Much more reliable, faster and less expensive substitutive methods to animal testing exist : organs on a chip, bioinformatics, bio-printing, stem cells, etc. must be used.
According to the European Commission’s Joint Research Center (JRC): “a strong dependence on animal experimentation can hinder the progress in certain areas of disease research”.
The new law (FDA Modernization Act 2.0), signed at the end of December 2022 by the American President, Joe Biden, authorizes to bring new drugs on the market without requiring data from animal experimentation. In other words, drug developers in the U.S. now have much more choice and less reliance on animal models ! They have the freedom to choose the most appropriate method for their application earlier in drug discovery, rather than being forced down an animal route.
In Germany, the NGO Arzte gegen Tierversuche has created a database, accessible on line, NAT (Non-Animal Technologies), which lists more than 250 research methods without animals ; tests developed around the world.
Saturday 22 of April, from 2 p.m. to 4 p.m., a Silent Line will take place on the bridge linking Strasbourg to Kehl to reveal these facts as well as those of the sale of primates by the University of Strasbourg.
The Silent Line is part of the International Day for the Abolition of animal experimentation (April 22) and World Animal Day in Laboratories (April 24).
This event is co-organized by 269 Life France, by the German association Black Forest for Animals and the Pro Anima Scientific Committee.






