Dr Nicolas Aznar — Nexpocan Project
Winner of the DVES Prize 2023 - Jury Prize Category
SES108 — March 2023Doctor in molecular and cellular biology and in cancer biology from the University of Lyon I, researcher at the Lyon Cancer Research Center, Nicolas Aznar is working on a new generation of organoids. In this interview, he explains us the advantages and challenges of his project Nexpocan.

Pro Anima Committee : The Pro Anima Scientific Committee and The Descroix-Vernier Foundation were absolutely delighted to award you for the 2023 edition of the Descroix-Vernier EthicScience Prize in the Jury Prize category for your project “NEXPOCAN. New generation of patient-derived organoids for better cancer modeling.” Could you present in a few words, for our readers, your project, its particularities, the objectives and results (in terms of and/or application) that you expect and within what time frame ?
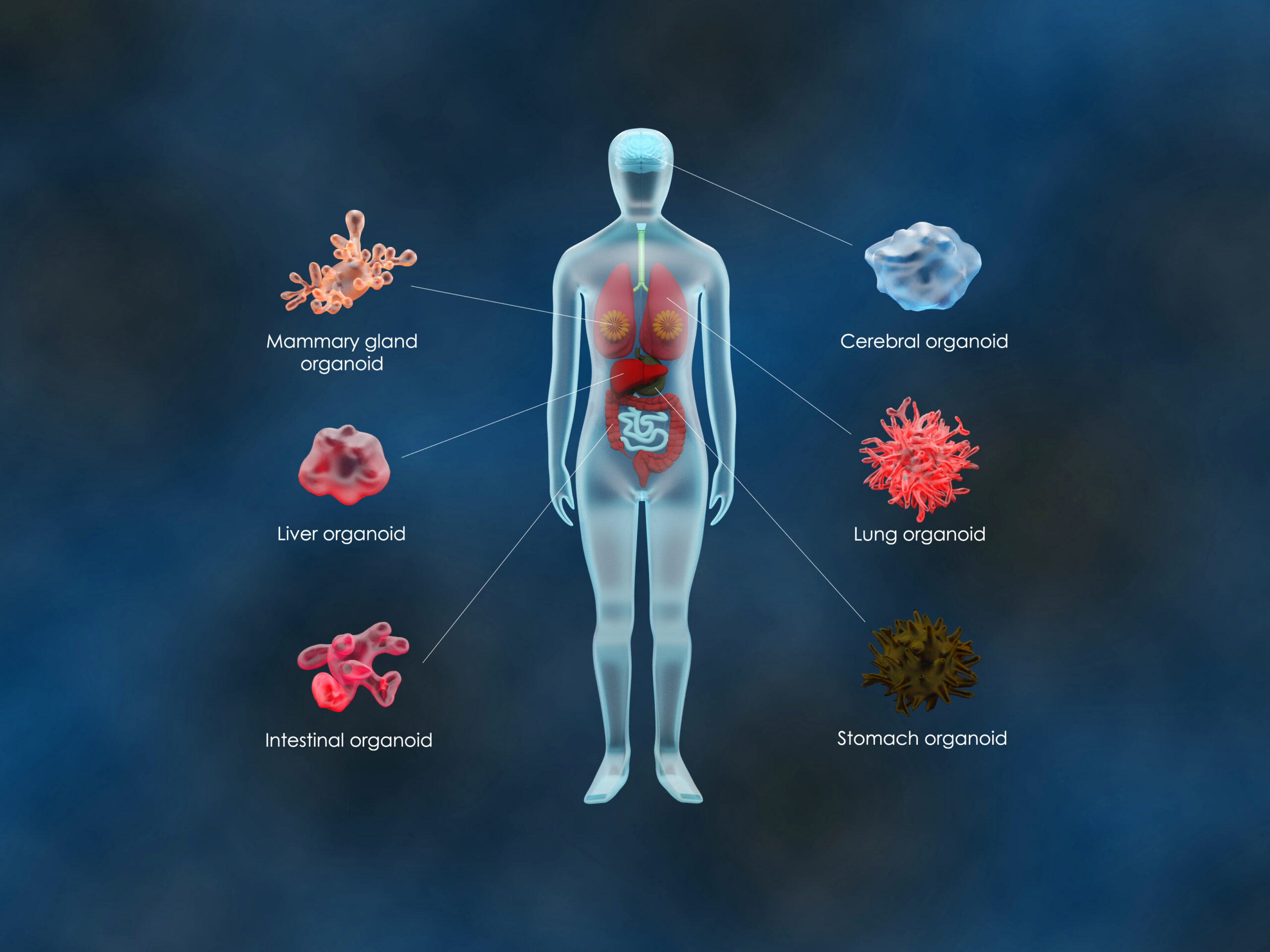
Dr. Nicolas Aznar : Stem cells (SC) and their culture in three dimensions (3D), also called organoids or mini-organs, offer promising prospects in the in vitro modeling of various pathologies such as cancer without resorting to the animal model. For example, from now on, the technological challenge with the use of organoids as a true “avatar” of the patient is to be able to amplify and cultivate them over the long term, while preserving the characteristics of the tissue of origin or of the primary tumor in the case of cancers.
However, although SCs and organoids are known to be very sensitive to their microenvironment, the lack of consideration or control of the latter by research teams constitutes strong constraints that could bias their results. To overcome this problem, researchers therefore need to find experimental conditions (standardized and more robust) allowing them to cultivate their organoids over the long term, while limiting the effects of biological drift.
NEXPOCAN project will therefore serve as a proof of concept on the feasibility of generating patient-derived organoid cultures in a standardized and more reliable manner to improve in the short term (1) the development of more effective personalized medicine to treat patients, but also in the longer term (2) the success rate in the development of anti-tumor drugs, thus opening the way to the implementation of preclinical trials without animals.
PA : What phase of your research the Prize will boost ?
NA : The Descroix-Vernier EthicScience Prize 2023 arrives at a pivotal period in the development of the NEXPOCAN project. Indeed, we are currently validating, using human samples, the culture devices that we have developed as well as the new environmental conditions conducive to SC that we have identified. It is a technological project, which is therefore not easy to finance through traditional calls for tenders. Thus obtaining the Descroix-Vernier EthicScience 2023 Prize will help us to support this project.

PA : The determining condition of the Descroix-Vernier EthicScience Prize is to promote and reward programs that use or develop non-animal models. What were your motivations, the reasons that have pushed you to work using those methods and towards the replacement of animal experimentation ?
NA : The development of drugs against cancer today has a dramatic failure rate of around 96% (including more than 90% during preclinical development). After such an observation, it is clear that animal models such as mice are not sufficiently predictive to evaluate the clinical effectiveness of drug candidates. Therefore, it was urgent for us to establish new, more ethical, robust and reliable models with the aim of more faithfully models of the morphological and molecular characteristics of pathologies such as cancer.
PA : Have you had to, at any point in your career, experiment on animals ? If so, what difficulties or failures have you encountered ?
NA : When you decided to embark on the optimization of organoid technology, we were in full confinement. During this period, patients were not tested against COVID 19 before surgery. For fear of contamination, we were therefore strictly prohibited from generating organoids from human samples. This is why we initially had to work with mouse organoids, which unfortunately are not always representative of the pathology that we want to study, with, sometimes, even responses to treatments which can be different compared to human organoids.
PA : In which research context (French, European, international) does your program fit ? What are the challenges of your project ?
NA : As part of a 3R approach (replace, reduce, refine), the NEXPOCAN project is based on the development of new 3D cell culture methods and is therefore part of a real desire to abolish in the medium term the use of animal models in research. Thus, we believe that the use of human organoid models will be accentuated in the years to come in order to meet future international ethical regulations. However, due to a lack of robustness, we observe a certain reluctance on the part of the private sector to use this new technology. The NEXPOCAN project will be developed in close collaboration not only with several companies in the health sector but also with several national and international platforms specialized in the field of 3D cell culture, in order to enable scientists from the public and private sector to carry out with greater reproducibility of organoid cultures. We therefore believe that NEXPOCAN will increase the performance and reliability of organoid technology and will meet the expectations of pharmaceutical companies so that they can routinely adopt this new alternative model to the use of animals.

Image credit : Pro Anima Committee – Brett Walch Photography – Nicolas Aznar


