Lab Monkeys and Big Pharma, Competitiveness of France for OoC, New NAMS database and more
News on non-animal methods
MARCH 25 - 29, 2024NEWS, REPORTS & POSITION STATEMENTS
1. Why Big Pharma won’t stop testing on lab monkeys
The long-tailed macaque is hardly the only species subjected to experiments in the name of biomedical science, but it’s among the most highly prized. Rodents, dogs, cats, rabbits and other animals are also in widespread use. But many experiments are typically performed on non-human primates, or NHPs, due to their relative genetic similarity with humans. Among other things, the animals are used to test the efficacy and toxicity of potential new medications. While the FDA recently began allowing non-animal toxicity testing, for the $1.4 trillion pharmaceutical industry, the long-tailed macaque remains the gold standard.
Experimenting on primates isn’t the only way to test new drugs. New and more humane methods are available. So when will Big Pharma move past monkeys ?
2. PEPR French plan : adequate to face intense international competition ?
Organs and organoids on chips, accelerators of drug development, are the subject of recently announced funding of 48 million euros over six years under the Priority Research Programs and Equipment (PEPR) launched by the French government as part of the France 2030 plan. Xavier Gidrol is co-directing this “exploratory” PEPR with counterparts from CNRS and Inserm. “One of the objectives is to create an industrial sector, an important step in terms of sovereignty.”
France is clearly not at the head of the pack, led by the United States and the China, and where “small” players like the Netherlands sometimes shine. Can the PEPR and its modest almost 50 million euros – the release of which could wait until the beginning of 2025 – help France play its part in the intense international competition underway ?
Read more (Le Monde, FR)
Learn more about PEPR (News NAMs 20/02/2024, news 11)
3. Organoids and OoC : towards new frontiers for preclinical studies
On February 22, research teams and industries from the region of Hauts-de-France who develop and/or use organoids and OoC models met to better understand and characterize new treatments or ingredients and avoid the use of animal models. The round table organised by Eurasanté took place in the presence of Anthony Treizebre from IEMN Lille, Cécile Legallais from UTC, Guillaume Vidal from GENFIT, Alexandre Martins from Sanofi, Anne Jouvenceau from the Health Innovation Agency/SGPI and Laurent Pineau – R&D Scientist in Biotechnology Lesaffre.
The round table highlighted among other things :
- The need to have access to industrial data and the results of clinical trials, whether positive or not ;
- The need for cross-functional training programs on new preclinical models, in order to provide a qualified workforce to meet the future needs of the industry ;
- In the field of agri-food, there is a growing demand for the use of these alternative technologies.
Read more (FR)
4. Beyond animal testing : The rise of organoids in toxicology research
The current demand in toxicology research is for a biological model that not only accelerates market entry but also conserves resources, without sacrificing the integrity of human-relevant data.
Patient-derived organoids (PDOs) emerge as a superior solution over traditional models. PDOs can be developed from both normal and diseased tissue and remain stable when expanded ex vivo in the lab, offering a distinct advantage for testing off-target and on-target toxicities within the same patient sample. Their adaptability for genetic modifications further positions them as an optimal tool for exploring the mechanisms of action of toxic compounds.
Read more (DDW)
5. Focus on animal-derived laboratory materials
The use of cell and tissue-based methods in basic, applied and regulatory science has been increasing exponentially. Animal-derived components, including serum, coating materials, growth factors and antibodies are routinely used in cell/tissue cultures and in general laboratory practices.
Tilo Weber and colleagues from the German Animal Welfare Federation have published a worldwide survey on animal-derived laboratory materials and reagents in scientific experimentation in 2022. This article, published in the journal Engineering in Life Sciences, is now one of the most downloaded of the journal.
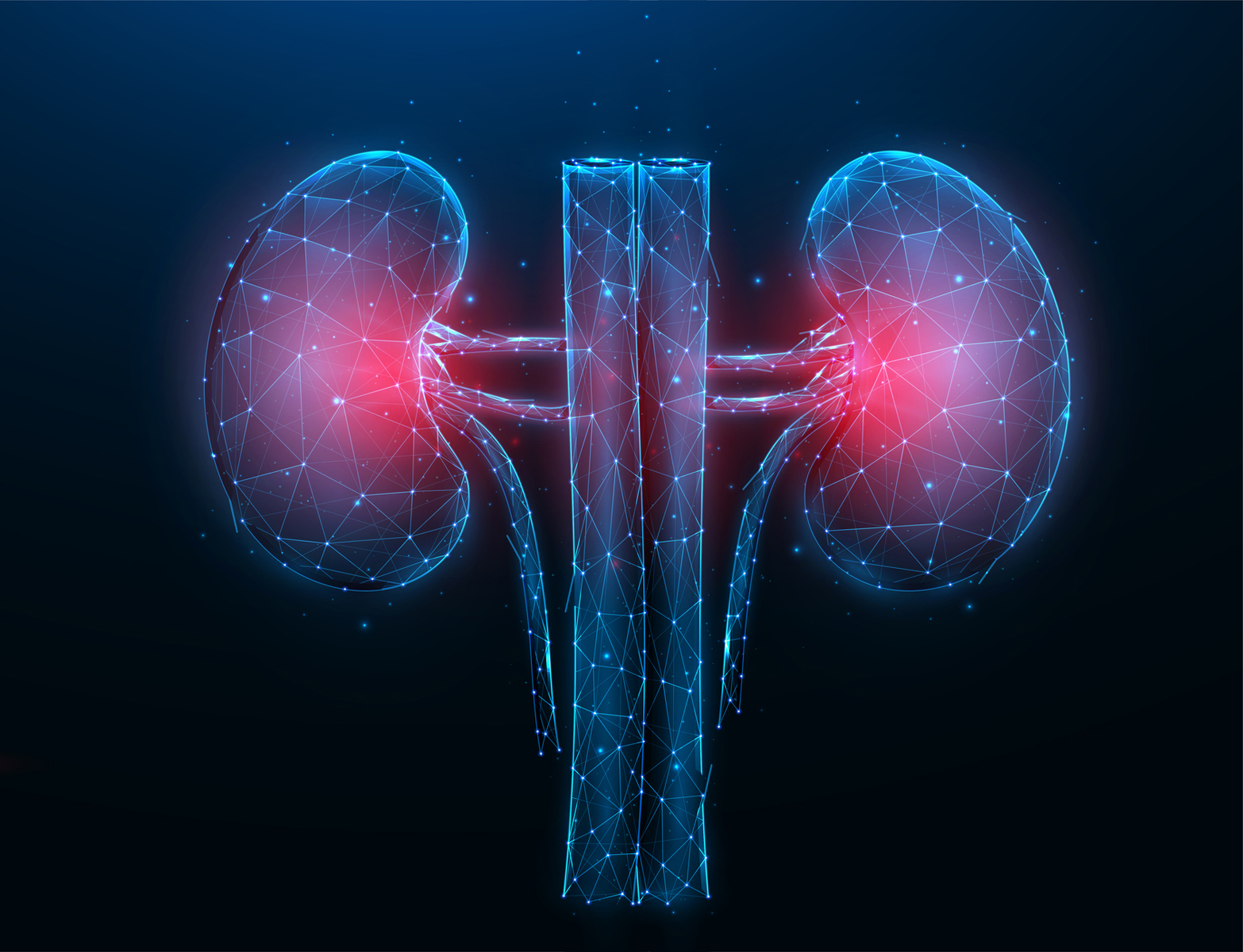
6. A kidney platform to reduce animal testing in tox studies
Researchers at the University of Washington (UW) schools of Medicine and Pharmacy, received the $7.3 million award from the National Center for Advancing Translational Sciences (NCATS — NIH) to advance drug development platforms that would reduce reliance on animal tests.
Their research aims to finalise multiple kidney-tissue platforms for drug testing, and submit those platforms to the US Food and Drug Administration (FDA) for approval. “We are incredibly pleased to receive this award. It’s an exciting project that will be the capstone for our already 10-plus years of NCATS funding for kidney-on-a-chip and kidney organoid research,” said Dr Jonathan Himmelfarb, Professor of Nephrology at the UW School of Medicine.
Read more (DDW)
NOMINATIONS
7. Professor Juergen Knoblich appointed new Head of Roche’s IHB
Earlier this week, Roche was pleased to announce Professor Juergen Knoblich, a pioneer in organoid biology, as the Head of its Institute of Human Biology.
Juergen’s developmental biology expertise and his work with cerebral organoids complement Roche’s focus on neurodegenerative diseases and bring academic research closer to industry application
See the announcement shared on Linkedin
TOOLS, PLATFORMS, CALLS
8. Search for NAMs : New database powered by QSAR Lab
Given the ethical, economic, and time-related challenges associated with animal testing, as well as emerging bans on such methods, it is paramount to develop and validate alternative methods, known as New Approach Methodologies (NAMs).
QSAR Lab has launched the first database of regulatory-relevant NAMs for assessing the safety of nano-materials and chemicals, from where regulators, academics & industry can extract state-of-the-art knowledge on different NAMs.
9. Unilever NAMs toolbox for DART safety
Developmental and reproductive toxicity (DART) refers to potential adverse effects that exposure to an ingredient may have on sexual function and fertility in women and men, as well as pre- and postnatal developmental toxicity in their offspring.
Unilever researchers have included additional measurements for DART-specific molecular initiating events in their in vitro NAMs pharmacological profiling panel. Using benchmark substances and exposure scenarios, researchers are currently evaluating their approach and in parallel, are working with external partners to address knowledge/data gaps.
10. A survey about readiness of computational NAMs for NGRA of chemicals
The European Partnership for the Assessment of Risks from Chemicals (PARC, co-funded by Horizon Europe) is conducting a survey on landscape and readiness of computational NAMs based on ML and AI approaches for NGRA of chemicals.
This survey examines whether and how ML and AI based QSAR models, and their predictions are suitable for regulatory applications, what are their main sources, and barriers to successful implementation.
SCIENTIFIC DISCOVERIES & PROTOCOLS
11. ‘A landmark moment’: scientists use AI to design antibodies from scratch
Researchers have used generative artificial intelligence (AI) to help them make completely new antibodies for the first time. The proof-of-principle work, reported this week in a preprint on bioRxiv, raises the possibility of bringing AI-guided protein design to the therapeutic antibody market, which is worth hundreds of billions of dollars. The work has not yet been peer reviewed.
Computational biochemist Joseph Watson, University of Washington, hopes this initial success will pave the way for the design of antibody drugs at touch of a button. “It feels like quite a landmark moment. It really shows this is possible.”
Read more (Nature)


