
Pro Anima presentation
Our engagement
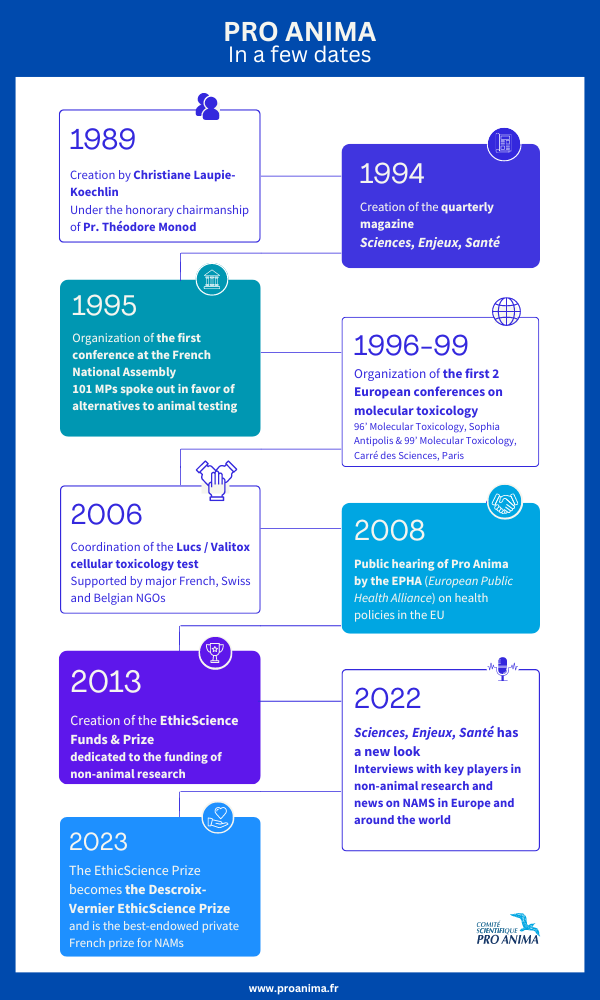
Created in 1989 under the honorary presidency of Professor Théodore Monod, the Scientific Committee Pro Anima is a council of scientists, academics and members of the medical profession, all volunteers, who work with laboratories to develop and promote research programs without experimenting on animals.
From the shortcomings and limits of the animal model, the Committee aims to promote human health safety through chemical substances that are better tested, more reliable for human health, more protective of the environment and without the use of animals.
« With technological progress and the advancement of scientific knowledge, it is possible to research and test chemicals differently and better than with animal experimentation »
Christiane Laupie-Koechlin
Founder — General Secretary
Our missions
The Pro Anima Scientific Committee :
- Raises awareness among citizens, political and economic actors ;
- Publishes a quarterly journal Sciences, Issues, Health ;
- Funds via the Descroix-Vernier EthicScience Prize research and alternative methods based on the most advanced knowledge and technologies.
The association is free from any political, philosophical, denominational or other subjection.
The Pro Anima Scientific Committee is currently chaired by Doctor Serge Kauffer.
Networks
Our history in a few dates
« Je préfère une ignorance malgré mon inlassable curiosité, à l’acquisition d’un savoir que je saurai n’avoir été obtenu que grâce à la souffrance d’un être vivant. »
Pr Théodore Monod


