
The uptake of NAMs
In recent years, the human and global health challenges of the 21st century (chemical and biological risks, cocktail effects, zoonoses, disease incidence) have highlighted the growing need for more reliable and effective biomedical research and toxicological tests.
In Human Health
New Approach Methodologies (NAMs), which do not use animals and focus on human data, are booming nationally and internationally. They are primarily based on in vitro or in silico models and often rely on artificial intelligence tools.
Generally based on knowledge of mechanisms of action and pathophysiological processes, they tend to offer greater reliability for humans and an increased understanding of diseases than traditional approaches. These advantages also have the potential to accelerate and reduce the cost of drug development.

In Chemical Risk Assessment
In addition to the fact that NAMs provide access to data collected on human models and thus help avoid the uncertainties associated with extrapolating animal tests to humans, they are likely to address the major challenge faced by assessment authorities in the face of the considerable number of chemical substances around us, which far exceed their capacity to characterize the hazards posed by these substances, alone or in mixtures. Given the time and resources required for animal testing, it is clear that they can no longer constitute the basis for risk assessment procedures and the resulting public health recommendations.

A Transition with Multiple Benefits
The transition to these predictive, relevant, and efficient models represents a real opportunity in terms of public and private research prospects, the evolution of expert assessment procedures, and health progress. This should generate economic, competitive, and sovereign benefits. In France, this opportunity needs to be more concretely supported.
Animal testing raises growing ethical, scientific, and societal concerns. Scientific reviews and evidence demonstrate that animal-based studies are unable to effectively predict outcomes in humans, due to a lack of translational value and reproducibility.
The scientific community and institutions recognize that between 80 and 99% of drugs approved for animal testing fail in human clinical trials. (The Flaws and Human Harms of Animal Experimentation / CNRS — PEPR MED-OOC / Analysis of animal-to-human translation).

In 2019, a study published in Drug Discovery Today highlighted organ-on-a-chip (OoC) as an emerging technology that could transform research and development efficiency and reduce costs by 10 to 26%.
The organ-on-a-chip market was valued at $116.5 million in 2023 and is expected to reach over $1.4 billion by 2032, driven by the growing demand for more accurate and reliable in vitro models of human organs for drug discovery, toxicity testing, and disease modeling.
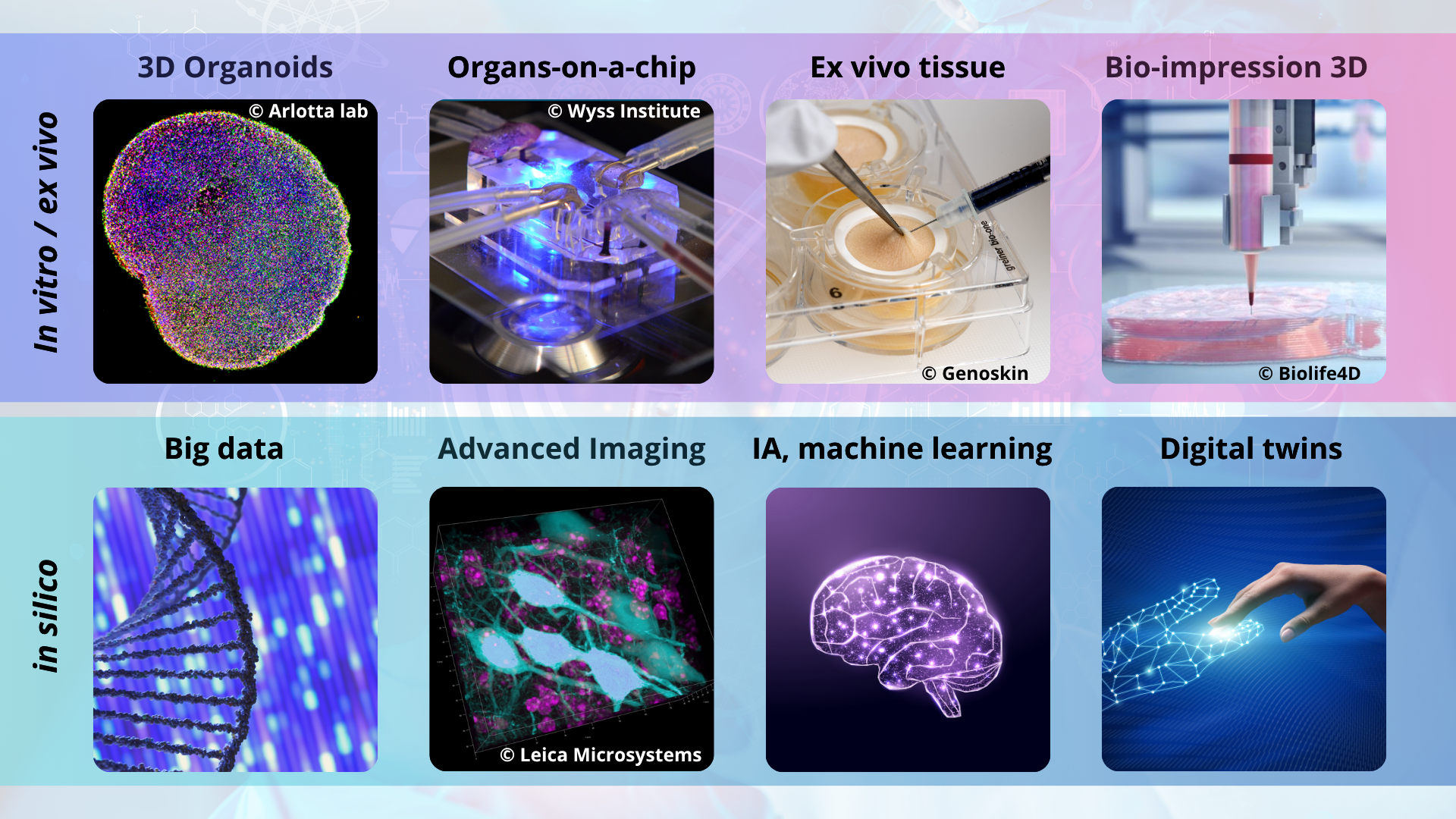
Significant decisions around the world to address these issues and their systemic nature
The most significant recent decisions have come from the United-States. In April 2025, the Food and Drug Administration (FDA) published its roadmap to improve public health by replacing animal testing in the development of monoclonal antibody therapies and other drugs. Implementation of this program began immediately for Investigational New Drug (IND) applications, where the inclusion of NAM data is encouraged, and is planned for a short timeframe of three to five years.
In parallel with this announcement, and to integrate these innovative human-centered sciences, the NIH announced the creation of the Office of Research Innovation, Validation, and Application (ORIVA) within the Office of the Director of the NIH. This new office will coordinate NIH-wide efforts to develop, validate, and expand the use of non-animal approaches across the agency’s biomedical research portfolio. It will also serve as a center for interagency coordination and regulatory translation to protect public health.
In Europe and France in particular, the current technological shift in life sciences, from traditional animal testing to microphysiological systems (MPS) and artificial intelligence, is clearly recognized as promising a new era of humane, precise, and ethical biomedical research.
Among the most advanced and structured European countries, the Netherlands serves as an example, notably with government funding from the National Groeifinds (NGF) in 2024 for the creation of the Centre for Animal-Free Biomedical Translation (CPBT), now called the Ombion Centre for Animal-Free Biomedical Translation.
This national center for the promotion and dissemination of animal-free innovations and know-how aims to improve and accelerate the translation of new biomedical innovations to patients and users, at a lower cost and without the use of animals.
The European Commission is currently working on a roadmap for a gradual phase-out of animal testing; roadmap which should be adopted by the first quarter of 2026.


