
The hard task of assessing endocrine disruptors
What if NAMs were the key?
Contribution by Dr. Jean-Pierre Cravedi, Toxicologist, former Director of Research at INRAE
The purpose of this article is not to revisit the subject of endocrine disruptors (EDs) in detail, as this topic has already been covered in a previous issue of the journal (SES115 — Dec. 2024), but rather to highlight the difficulties inherent in these substances when it comes to assessing the risk they pose to the population. Although EDCs also pose a challenge in terms of their environmental impact, we will only address the human health aspect of the issue here.
It should be remembered that assessing the risks associated with a substance requires knowledge of both exposure to that substance and its toxicity (hazard) to the exposed organism. While determining exposure to EDs is no different from other types of substances, the same cannot be said for their harmful effects. Data collected over the past 30 years show that these effects have some peculiarities in terms of dose-response relationships, delayed toxic effects, and synergistic effects. In addition to these observations, which are not necessarily unique to EDs, there are questions about the relevance of animal models for assessing such effects.
The effects of low-dose exposure
The effects of EDs can occur at very low doses, which is not surprising since they often mimic hormones that have the property of acting at very low concentrations. To take just one example, bisphenol A (BPA), a synthetic substance used in particular in plastics manufacturing (especially for polycarbonate plastics) and classified on the list of substances of very high concern by the European Chemicals Agency (ECHA) since 2017 due to its endocrine-disrupting properties responsible for severe effects on human health. Based on the toxicological data available at the time, the European Food Safety Authority (EFSA) established a tolerable daily intake (TDI) of 50 µg/kg body weight/day in 2006, concluding that below this exposure threshold, BPA posed no health risk to the general population, given the estimated exposure levels (EFSA, 20061). Due to additional toxicological data not directly related to endocrine disruption, the TDI was revised downward by EFSA in 20152 to 4 µg/kg body weight/day. A new opinion, published in April 2023 by EFSA, set the TDI at 0.0002 µg/kg body weight/day, which is 250,000 times lower than the value calculated 17 years earlier (EFSA, 2023 3). This considerable difference is due to the inclusion of targets that had not been examined previously (immune system and inflammation), the integration of more human data into the evaluation process, and a better understanding of the mechanisms of action of BPA at the cellular and molecular level. While the immune system was ultimately identified by EFSA experts as the target most sensitive to the effects of BPA, toxicity to development and reproduction, as well as to metabolism, directly related to the ED properties of BPA, was also observed at doses several thousand times lower than those that caused effects on the liver or kidneys in animals, which served as the basis for the EFSA’s 2015 opinion. This very low-dose effect of BPA, due to various interferences with hormonal regulatory mechanisms in several species, was described more than twelve years ago by certain scientists (vom Saal FS, Hughes, 20054; Vandenberg et al. 20135). Finally, based on this new TDI and the available exposure data, in 2023 the EFSA warned that dietary exposure to BPA was a health concern for all age groups in the general population.
Non-monotonic dose-response curves
Another peculiarity related to dosage concerns non-monotonic dose-response curves, which have been highlighted in several experimental studies on EDs. Toxicologists often say that it is the dose that makes the poison, in other words that the effects are more pronounced when the toxic substance is administered in high doses. When these results are plotted on a graph, we refer to a monotonic curve, which makes it easy to derive a dose with no toxic effect and thus propose a threshold (no-effect dose, NED) below which there is no risk (shown in Figure 1).
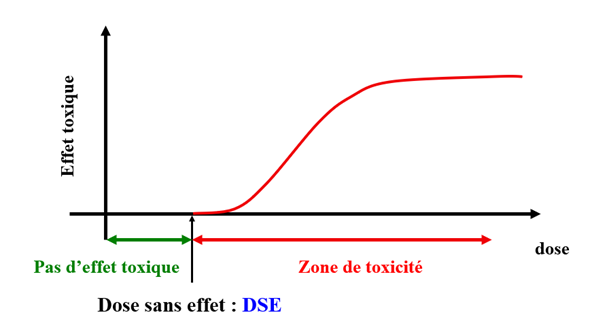
Figure 1: Determination of the no-effect dose based on the administration of decreasing doses of a substance to an organism. Credits: Prof. Jean-Pierre Cravedi
However, the effects caused by certain EDs do not always seem to be related to the dose received by an individual. Some effects appear at low doses, then decrease as doses increase, and increase again at higher doses, resulting in U‑shaped dose-response curves (non-monotonic dose-response relationship) and calling into question the usual methods of risk assessment (Figure 2). In this case, it becomes much more difficult to determine a dose without effect. Although these non-monotonic dose-response curves have been described for more than 10 years for several EDs, suggesting that this type of curve was more common for EDs than for other toxic agents (Vandenberg et al., 20126), they have been the subject of much recent debate in the scientific community and risk assessment agencies (Lorenz et al., 20127; Lagarde et al., 20158; Hill et al., 20189). Many experts concluded that EDs could give rise to non-monotonic dose-response curves, but that the frequency of the event was less than suggested by Vandenberg et al. (2012)10.
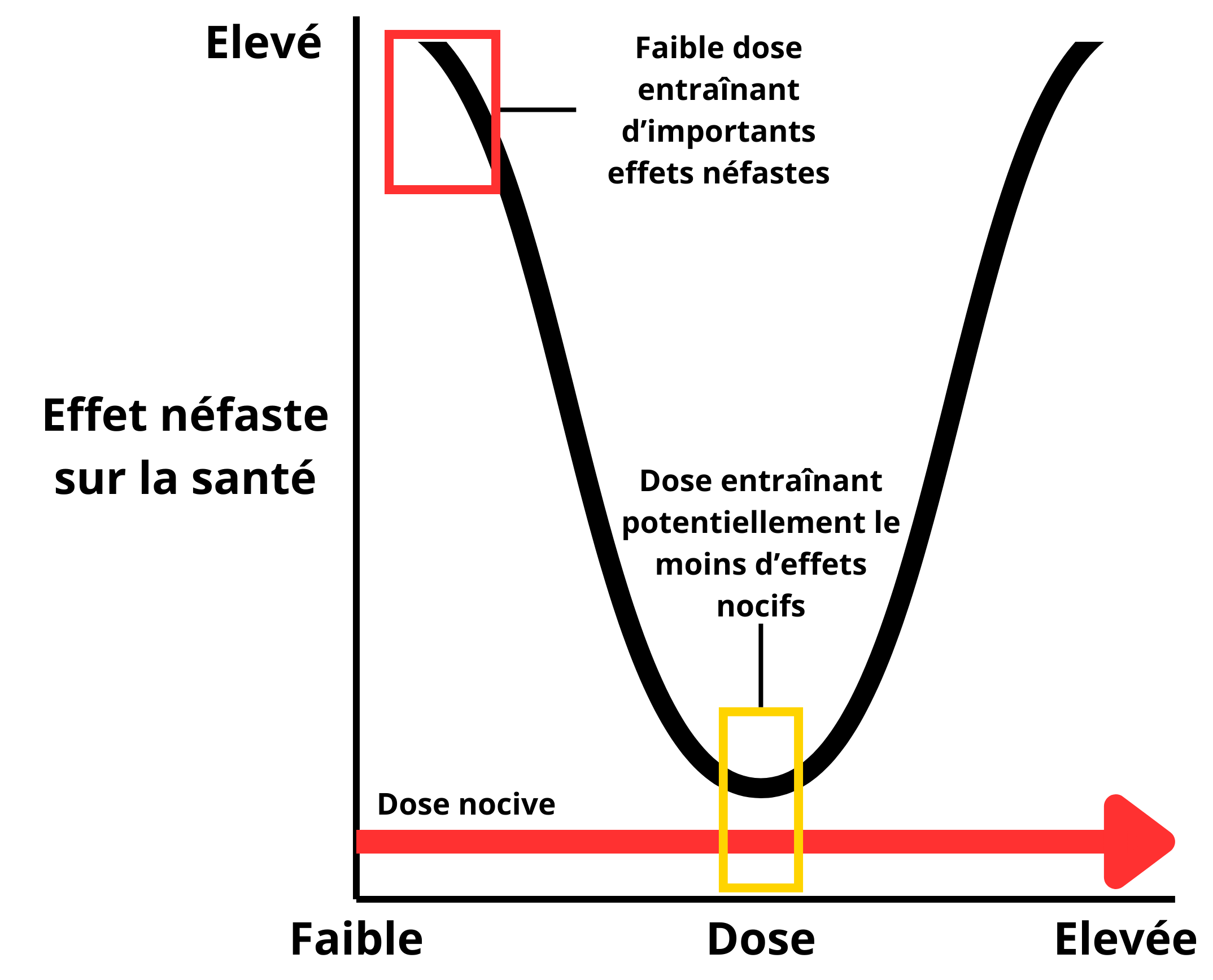
Figure 2: Non-monotonic dose-response curve (U‑shaped). In this case, toxic effects may occur at low doses, whereas they may be less pronounced at medium doses. Adapted from Vandenberg et al. 201211.
Today, health agencies acknowledge the plausibility of this phenomenon for several EDs, and some (ANSES, EFSA) propose the use of NAMs to incorporate this singularity into risk assessment (Lagarde et al., 201512; Chevillotte et al., 2017a,b 13, 14; EFSA, 202115).
Vulnerability “windows”
When it comes to EDs, there is no doubt that the period of exposure, rather than the dose or duration of exposure, plays a decisive role in the adverse effects they produce. The most sensitive exposure “window” for most EDs is the perinatal period, i.e., fetal life and early childhood. This is the source of most of the effects attributed to certain phthalates or bisphenols, for example. To take the example of diethylstilbestrol (or DES) mentioned in the previous article, this drug, which was once prescribed to prevent miscarriages and pregnancy complications, is responsible for gynecological cancers in women exposed in utero between the 6th and 17th weeks of pregnancy. However, no increased risk of this type of cancer was observed in mothers treated with this drug during pregnancy. It has been shown that the incidence of vaginal adenocarcinoma in women exposed to diethylstilbestrol in utero decreased when treatment was prescribed later in pregnancy (O’Brien, 197916, Hoover et al., 201117).
Puberty is also a period of vulnerability to EDs. In this context, the concept of lifelong exposure, which prevails in chronic exposure risk assessment models, loses much of its relevance.
Delayed effects
In addition to the fact that there is a window of vulnerability with regard to EDs, it has been discovered that the harmful effects of EDs can appear long after exposure has occurred. Among the many examples of these delayed effects is that of dioxin, following the Seveso accident in Italy in 1976. Dioxin is a powerful endocrine disruptor that is persistent and slowly eliminated in breast milk in mammals. Monitoring of exposed populations in the Seveso area showed that perinatal exposure to dioxin could lead to a significant decline in sperm quality in adulthood, and in particular a 50% reduction in the number of spermatozoa per mL of semen (Figure 3) (Mocarelli et al., 201118).
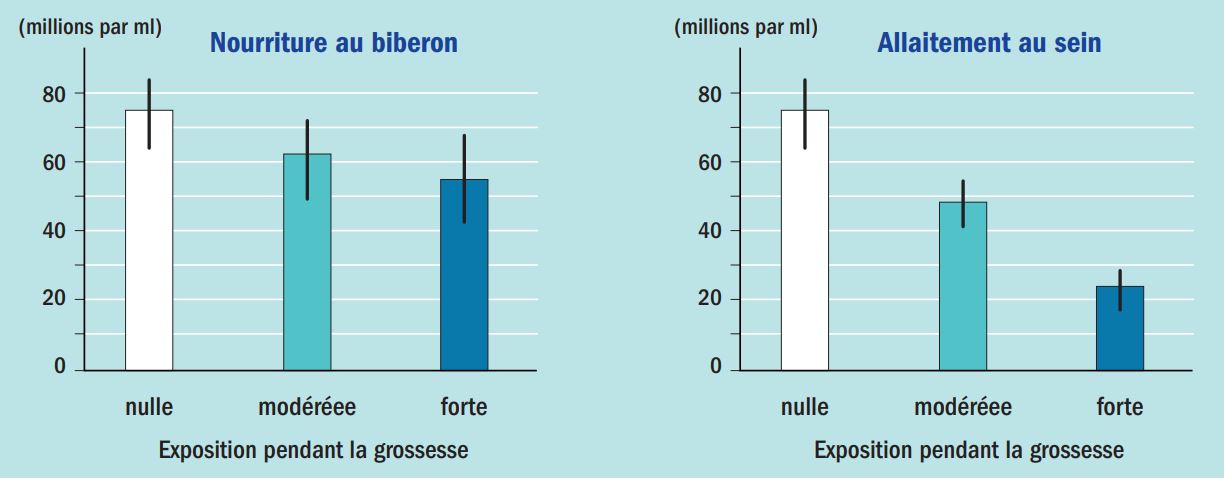
Figure 3: Effect of perinatal exposure to dioxin on sperm count. Exposure levels are classified as low, moderate, or high based on estimates of dioxin concentrations in mothers’ serum at the time of the accident. According to Habert R. 202119.
While the effects are minor or insignificant for bottle-fed infants, even when their mothers were heavily exposed, the results become very significant in the case of breastfeeding. These data demonstrate the irreversible effect of an ED occurring more than 30 years after exposure.
Transgenerational impacts
Further research on the health effects of diethylstilbestrol mentioned earlier shows impacts not only on the children of treated women, but also on their grandchildren (increased risk of malformations). These observations suggest multi- or transgenerational impacts of EDs, i.e., observed in individuals who have never been in contact with the drug or toxic substance. Some experimental work conducted on rodents with EDs such as vinclozolin, a now-banned fungicide, bisphenol A, and certain phthalates shows that the effects observed in exposed individuals can be transmitted across several generations (Brehm & Flaws, 2019 20). Although not yet fully understood, the mechanisms involved are probably epigenetic, i.e., involving changes in the environment or gene expression without changing the DNA sequence (without the appearance of mutations).
The effects of mixtures
EDs “cocktails” can give rise to synergistic or potentiating effects, meaning that the effect of the mixture can be greater than that of each of its components taken individually. While this phenomenon is not specific to EDs, it has been particularly highlighted in this case because of the multiple targets on which these molecules can act. Among several examples of interactions between EDs resulting in greater toxicity of the mixture compared to the sum of the effects of each of the constituents, we can cite an experimental study in rats comparing the effects on sperm concentration of two EDs, vinclozolin (described above) and genistein (a phytoestrogen found in soybeans) tested at different daily doses for 80 days from the in utero period to adulthood. The results of the study indicate that the most pronounced effect (a nearly 50% decrease in sperm count) was observed for the mixture of genistein (dose of 1mg/kg) + vinclozolin (dose of 1mg/kg), while no effect was observed when each substance was tested individually at the same concentrations (Figure 4). (Eustache et al., 200921).
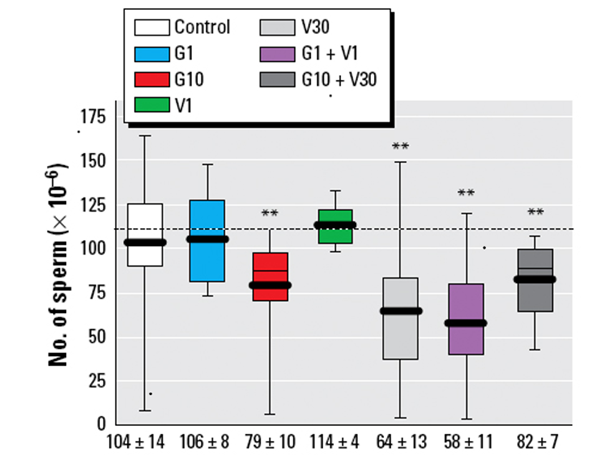
Figure 4: Effect on sperm quality of chronic individual or combined exposure to low doses of genistein (G1, G10) and vinclozolin (V1, V30) in rats. According to Eustache et al., (2009)22.
French researchers have explored the mechanisms behind these synergies. They demonstrated that two EDs can bind simultaneously to the same receptor in the cell nucleus, thereby increasing the quantity of bound molecules and creating a synergistic effect that disrupts the regulation of various physiological functions (Delfosse et al., 201523). Extending this work to other mixtures and a pair of receptors indicates that mixtures of three EDCs can greatly increase the synergy of a binary mixture and that certain receptors can interact with each other and amplify the synergy observed with a single receptor (Delfosse et al., 2021 24). In the coming years, combining these molecular screening methods on receptors with artificial intelligence (AI) should make it possible to more effectively predict the cocktail effects that pose the greatest risk to health.
The relevance of animal models questioned
Risk assessment is generally based on data from animal experiments, which are used to determine a no-effect dose. However, in endocrinology, it is well known that there are significant differences between species. The scientific literature reports numerous examples of these differences, and experts frequently question the validity of extrapolating data collected from animals to humans. With regard to the issue of EDs, it is probably René Habert’s team that has most attracted the attention of assessment bodies to the uncertainties associated with these extrapolations. For example, this team was able to show that human fetal testicles were 100 times more sensitive to bisphenol A than fetal testicles in rats or mice. Conversely, using the same experimental model, diethylstilbestrol has no effect in humans, whereas it slows sperm development and reduces testosterone production in mice and rats. As for their studies on phthalates, they highlight a reduction in the development of future sperm during fetal life in both humans and rats, but a decrease in testicular testosterone production only in rats and not in humans (Habert et al., 2014 25).
All of this work has shown, on the one hand, that transposing animal results to humans introduces an additional (and undoubtedly unnecessary) level of complexity and, on the other hand, that certain pathways of toxicity for humans cannot be tested on animals. In Europe, several initiatives have emerged to improve the diagnosis of diseases and dysfunctions related to EDs, without resorting to animal testing. These include the Dutch AFARA (Animal-Free Assays for Endocrine Disruption – from Science to Regulatory Acceptance) project, which began in 2023 and involves several French teams. This project aims to develop the use of animal-free models for identifying EDs and to facilitate the acceptance of these models for risk assessment.
EDs and NAMs: are they compatible?
The specific characteristics mentioned above and the complexity of the mechanisms involved in endocrine disruption make it difficult to characterize their long-term adverse effects and call into question the principles of conventional toxicology. More than other categories of substances, EDs require new approaches to assess their risk. Among these approaches, the use of data from NAM to enable faster assessment, better understanding of toxicity mechanisms, and prioritization of assessments is recommended by several authors and agencies (Barton-McLaren, 202226; Svingen et al., 202227; US-EPA, 202328). These methods include the development of in silico models (computational toxicology), tests based on molecular, cellular, or tissue models (organoids or organs-on-a-chip), most often in combination and relying on bioinformatics and systems biology tools to effectively extract information and promote an integrative approach. They represent an increasingly comprehensive and effective arsenal for responding to the health challenges posed by EDs.
In the case of EDs, however, there is a major challenge to replacing traditional toxicology approaches with NAMs. This challenge stems from the regulatory definition of EDs, which requires that three criteria be met for a substance to be considered an ED:
- an adverse effect on an intact organism or its offspring;
- endocrine activity (or an endocrine mode of action);
- the adverse effect is a consequence of the endocrine mode or mechanism of action.
Because of these classification criteria, only a limited number of substances are currently identified as EDs. If we want to rely exclusively on AMMs to assess the risk of EDs, the criterion of “adverse effect on an intact organism” poses a problem and is difficult to meet without resorting to in vivo tests (or a battery of tests). Without a revision of the current regulatory criteria, it is therefore likely that, initially, alternative methods will only be used to meet criteria 2 and 3 and, to a greater extent, to select substances for priority study. However, it cannot be ruled out that this challenge will lead to a review of the regulatory criteria for EDs (Svingen, 202229).
These obstacles do not prevent research from progressing and proposing alternatives to the methods currently used to characterize the danger of EDs and assess the health risk they pose to populations. A group of researchers involved in the European PARC project, to which the Pro Anima Committee contributes, recently published a plea in favor of NAMs in the identification and characterization of EDs involved in metabolic disruption. This article provides an overview of the alternative approaches and methods developed in the PARC project and shows how they will contribute to the identification of EDs and the elucidation of their mechanism of action (Braeuning, 202330).
About the author
Dr Jean-Pierre Cravedi

Toxicologist, Chairman of the Scientific Council of Aprifel, former expert at ANSES and EFSA.
This former INRAE research director headed the Xenobiotics Joint Research Unit in Toulouse before becoming deputy head of the Human Nutrition Department from 2014 to 2019. His work has led him to study the fate and effects of several contaminants present in the environment or in food, including endocrine disruptors.
Dr. Jean-Pierre Cravedi has also been chairman of the strategic committee of the Descroix-Vernier EthicScience Prize since 2025.
References
* NAM = New Approach Methodologies
(1) EFSA, Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to 2,2‑BIS(4‑HYDROXYPHENYL)PROPANE, EFSA Journal 2006; EFSA Journal. https://doi.org/10.2903/j.efsa.2007.428.
(2) EFSA, Scientific Opinion on the Risks to Public Health Related to The Presence of bisphenol A (BPA) in foodstuffs, EFSA Journal 2015, 13(1):3978, 1040 pp.
(3) EFSA, Scientific opinion on the re-evaluation of the risks to public health related to the Presence of Bisphenol A (BPA) in Foodstuffs, EFSA Journal 2023, 21(4):6857, 392 pp.
(4) vom Saal FS, Hughes C. Bisphenol A: vom Saal and Hughes Respond, Environ Health Perspect 2006 Jan., 114(1):A16 – 7.
(5) Vandenberg LN, Ehrlich S, Belcher SM, Ben-Jonathan N, Dolinoy DC, Hugo ER, Hunt PA, Newbold RR, Rubin BS, Saili KS, Soto AM, Wang HS, & vom Saal FS, Low dose effects of bisphenol A, Endocrine Disruptors 2013, 1:1. DOI: 10.4161/endo.26490.
(6) Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, et al., Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose-responses, Endocr Rev 2012, 33, 378 – 455 pp. 10.1210/er.2011 – 1050.
(7) Lorenz R, Rhomberg LR, Goodman JE. Low-dose effects and nonmonotonic dose – responses of endocrine disrupting chemicals: Has the case been made?, Regulatory Toxicology and Pharmacology 2012, 64:1, 130 – 133 pp.
(8) Lagarde, F., Beausoleil, C., Belcher, S.M. et al. Non-monotonic dose-response relationships and endocrine disruptors: a qualitative method of assessment, Environ Health 2015, 14:13. https://doi.org/10.1186/1476 – 069X-14 – 13.
(9) Hill CE, Myers JP, Vandenberg LN, Nonmonotonic Dose-Response Curves Occur in Dose Ranges That Are Relevant to Regulatory Decision-Making, Dose Response 2018 Sep., 13;16(3):1559325818798282. DOI: 10.1177/1559325818798282.
(10) Chevillotte G, Bernard A, Varret C, Ballet P, Bodin L, and Roudot AC, 2017a, Probabilistic assessment method of the non-monotonic dose-responses-Part I: methodological approach. Food and Chemical Toxicology 2017, 106, 376 – 385 pp.
(11) Chevillotte G, Bernard A, Varret C, Ballet P, Bodin L, and Roudot AC, 2017b, Probabilistic assessment method of the non-monotonic dose-responses-Part II: robustness assessment, Food and Chemical Toxicology 2017, 110, 214 – 228 pp.
(12) EFSA, Scientific Committee, Opinion on the Impact of non-monotonic Dose Responses on EFSA’s Human Health Risk Assessments, EFSA Journal 2021, 19:10:e06877.
(13) O’Brien PC, Nouer KL, Robboy SJ, Bames AB, Kaufman RH, Tilley BC, Vaginal epithelial changes in young human enrolled in the National Cooperative Diethylstilbestrol Adenosis (DESAD) project. Obstet Gynecol l979, 43: 300 – 8.
(14) Hoover, R.N.; Hyer, M.; Pfeiffer, R.M.; Adam, E.; Bond, B.; Cheville, A.L.; Colton, T.; Troisi, R. Adverse Health Outcomes in Women Exposed in Utero to Diethylstilbestrol. N. Engl. J. Med. 2011, 365, 1304 – 1314.
(15) Mocarelli P, Gerthoux PM, Needham LL, Patterson DG Jr, Limonta G, Falbo R, Signorini S, Bertona M, Crespi C, Sarto C, Scott PK, Turner WE, Brambilla P, Perinatal exposure to low doses of dioxin can permanently impair human semen quality, Environ Health Perspect 2011 May, 119:5, 713 – 8.
(16) Habert R., Les perturbateurs endocriniens : des toxiques spécifiques ; adsp n° 115 septembre 2021.
(17) Brehm E, Flaws JA, Transgenerational Effects of Endocrine-Disrupting Chemicals on Male and Female Reproduction, Endocrinology 2019, 160:6, 1421 – 1435 pp.
(18) Eustache F, Mondon F, Canivenc-Lavier MC, et al. Chronic dietary exposure to a low-dose mixture of genistein and vinclozolin modifies the reproductive axis, testis transcriptome, and fertility. Environ Health Perspect. 2009;117(8):1272 – 1279. doi:10.1289/ehp.0800158
(19) Delfosse V, Dendele B, Huet T, Grimaldi M, Boulahtouf A, Gerbal-Chaloin S, Beucher B, Roecklin D, Muller C, Rahmani R, Cavaillès V, Daujat-Chavanieu M, Vivat V, Pascussi JM, Balaguer P, Bourguet W, Synergistic activation of human pregnane X receptor by binary cocktails of pharmaceutical and environmental compounds, Nat Commun 2015 Sep., 3;6:8089.
(20) Delfosse V, Huet T, Harrus D, Granell M, Bourguet M, Gardia-Parège C, Chiavarina B, Grimaldi M, Le Mével S, Blanc P, Huang D, Gruszczyk J, Demeneix B, Cianférani S, Fini JB, Balaguer P, Bourguet W, Mechanistic insights into the synergistic activation of the RXR-PXR heterodimer by endocrine disruptor mixtures, Proc Natl Acad Sci USA 2021 Jan., 5;118(1):e2020551118.
(21) Habert R, Muczynski V, Grisin T, et al., Concerns about the widespread use of rodent models for human risk assessments of endocrine disruptors, Reproduction 2014, 147(4), 119 – 29.
(22) Barton-Maclaren TS, Wade M, Basu N, Bayen S, Grundy J, Marlatt V, Moore R, Parent L, Parrott J, Grigorova P, Pinsonnault-Cooper J, Langlois VS, Innovation in regulatory approaches for endocrine disrupting chemicals: The journey to risk assessment modernization in Canada, Environmental Research 2022, Part C, 204:112225.
(23) Svingen T, Schwartz CL, Rosenmai AK, Ramhøj L, Johansson HKL, Hass U, et al., Using Alternative Test Methods to Predict Endocrine Disruption and Reproductive Adverse Outcomes: Do We Have Enough Knowledge?, Environ. Pollut. 2022, 304:119242.
(24) U.S. Environmental Protection Agency, Availability of New Approach Methodologies (NAMs) in the Endocrine Disruptor Screening Program (EDSP), EPA 2023, EPA-HQ-OPP-2021 – 0756.
(25) Svingen T, Endocrine Disruptors in a New Era of Predictive Toxicology and Dealing With the “More is Different” Challenge, Front Toxicol 2022 Apr, 27;4:900479.
(26) Braeuning A, Balaguer P, Bourguet W, Carreras-Puigvert J, Feiertag K, Kamstra JH, Knapen D, Lichtenstein D, Marx-Stoelting P, Rietdijk J, Schubert K, Spjuth O, Stinckens E, Thedieck K, van den Boom R, Vergauwen L, von Bergen M, Wewer N and Zalko D, Development of new approach methods for the identification and characterization of endocrine metabolic disruptors — a PARC project, Front. Toxicol 2023, 5:1212509.
- EFSA, Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to 2,2‑BIS(4‑HYDROXYPHENYL)PROPANE, EFSA Journal 2006; EFSA Journal. https://doi.org/10.2903/j.efsa.2007.428.
- EFSA, Scientific Opinion on the Risks to Public Health Related to The Presence of bisphenol A (BPA) in foodstuffs, EFSA Journal 2015, 13(1):3978, 1040 pp.
- EFSA, Scientific opinion on the re-evaluation of the risks to public health related to the Presence of Bisphenol A (BPA) in Foodstuffs, EFSA Journal 2023, 21(4):6857, 392 pp.
- vom Saal FS, Hughes C. Bisphenol A: vom Saal and Hughes Respond, Environ Health Perspect 2006 Jan., 114 (1):A16 – 7.
- Vandenberg LN, Ehrlich S, Belcher SM, Ben-Jonathan N, Dolinoy DC, Hugo ER, Hunt PA, Newbold RR, Rubin BS, Saili KS, Soto AM, Wang HS, & vom Saal FS, Low dose effects of bisphenol A, Endocrine Disruptors 2013, 1:1. DOI: 10.4161/endo.26490.
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, et al., Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose-responses, Endocr Rev 2012, 33, 378 – 455 pp. 10.1210/er.2011 – 1050.
- Lorenz R, Rhomberg LR, Goodman JE. Low-dose effects and nonmonotonic dose – responses of endocrine disrupting chemicals: Has the case been made?, Regulatory Toxicology and Pharmacology 2012, 64:1, 130 – 133 pp.
- Lagarde, F., Beausoleil, C., Belcher, S.M. et al. Non-monotonic dose-response relationships and endocrine disruptors: a qualitative method of assessment, Environ Health 2015, 14:13. https://doi.org/10.1186/1476 – 069X-14 – 13.
- Hill CE, Myers JP, Vandenberg LN, Nonmonotonic Dose-Response Curves Occur in Dose Ranges That Are Relevant to Regulatory Decision-Making, Dose Response 2018 Sep., 13;16(3):1559325818798282. DOI: 10.1177/1559325818798282.
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, et al., Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose-responses, Endocr Rev 2012, 33, 378 – 455 pp. 10.1210/er.2011 – 1050.
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, et al., Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose-responses, Endocr Rev 2012, 33, 378 – 455 pp. 10.1210/er.2011 – 1050.
- Lagarde, F., Beausoleil, C., Belcher, S.M. et al. Non-monotonic dose-response relationships and endocrine disruptors: a qualitative method of assessment, Environ Health 2015, 14:13. https://doi.org/10.1186/1476 – 069X-14 – 13.
- Chevillotte G, Bernard A, Varret C, Ballet P, Bodin L, and Roudot AC, 2017a, Probabilistic assessment method of the non-monotonic dose-responses-Part I: methodological approach. Food and Chemical Toxicology 2017, 106, 376 – 385 pp.
- Chevillotte G, Bernard A, Varret C, Ballet P, Bodin L, and Roudot AC, 2017b, Probabilistic assessment method of the non-monotonic dose-responses-Part II: robustness assessment, Food and Chemical Toxicology 2017, 110, 214 – 228 pp.
- EFSA, Scientific Committee, Opinion on the Impact of non-monotonic Dose Responses on EFSA’s Human Health Risk Assessments, EFSA Journal 2021, 19:10:e06877.
- O’Brien PC, Nouer KL, Robboy SJ, Bames AB, Kaufman RH, Tilley BC, Vaginal epithelial changes in young humans enrolled in the National Cooperative Diethylstilbestrol Adenosis (DESAD) project. Obstet Gynecol l979, 43: 300 – 8.
- Hoover, R.N.; Hyer, M.; Pfeiffer, R.M.; Adam, E.; Bond, B.; Cheville, A.L.; Colton, T.; Troisi, R. Adverse Health Outcomes in Women Exposed in Utero to Diethylstilbestrol. N. Engl. J. Med. 2011, 365, 1304 – 1314.
- Mocarelli P, Gerthoux PM, Needham LL, Patterson DG Jr, Limonta G, Falbo R, Signorini S, Bertona M, Crespi C, Sarto C, Scott PK, Turner WE, Brambilla P, Perinatal exposure to low doses of dioxin can permanently impair human semen quality, Environ Health Perspect 2011 May, 119:5, 713 – 8.
- Habert R., Endocrine disruptors: specific toxins; adsp no. 115 September 2021.
- Brehm E, Flaws JA, Transgenerational Effects of Endocrine-Disrupting Chemicals on Male and Female Reproduction, Endocrinology 2019, 160:6, 1421 – 1435 pp.
- Eustache F, Mondon F, Canivenc-Lavier MC, et al. Chronic dietary exposure to a low-dose mixture of genistein and vinclozolin modifies the reproductive axis, testis transcriptome, and fertility. Environ Health Perspect. 2009;117(8):1272 – 1279. doi:10.1289/ehp.0800158
- Eustache F, Mondon F, Canivenc-Lavier MC, et al. Chronic dietary exposure to a low-dose mixture of genistein and vinclozolin modifies the reproductive axis, testis transcriptome, and fertility. Environ Health Perspect. 2009;117(8):1272 – 1279. doi:10.1289/ehp.0800158
- Delfosse V, Dendele B, Huet T, Grimaldi M, Boulahtouf A, Gerbal-Chaloin S, Beucher B, Roecklin D, Muller C, Rahmani R, Cavaillès V, Daujat-Chavanieu M, Vivat V, Pascussi JM, Balaguer P, Bourguet W, Synergistic activation of human pregnane X receptor by binary cocktails of pharmaceutical and environmental compounds, Nat Commun 2015 Sep., 3;6:8089.
- Delfosse V, Huet T, Harrus D, Granell M, Bourguet M, Gardia-Parège C, Chiavarina B, Grimaldi M, Le Mével S, Blanc P, Huang D, Gruszczyk J, Demeneix B, Cianférani S, Fini JB, Balaguer P, Bourguet W, Mechanistic insights into the synergistic activation of the RXR-PXR heterodimer by endocrine disruptor mixtures, Proc Natl Acad Sci USA 2021 Jan., 5;118(1):e2020551118.
- Habert R, Muczynski V, Grisin T, et al., Concerns about the widespread use of rodent models for human risk assessments of endocrine disruptors, Reproduction 2014, 147(4), 119 – 29.
- Barton-Maclaren TS, Wade M, Basu N, Bayen S, Grundy J, Marlatt V, Moore R, Parent L, Parrott J, Grigorova P, Pinsonnault-Cooper J, Langlois VS, Innovation in regulatory approaches for endocrine disrupting chemicals: The journey to risk assessment modernization in Canada, Environmental Research 2022, Part C, 204:112225.
- Svingen T, Schwartz CL, Rosenmai AK, Ramhøj L, Johansson HKL, Hass U, et al., Using Alternative Test Methods to Predict Endocrine Disruption and Reproductive Adverse Outcomes: Do We Have Enough Knowledge?, Environ. Pollut. 2022, 304:119242.
- U.S. Environmental Protection Agency, Availability of New Approach Methodologies (NAMs) in the Endocrine Disruptor Screening Program (EDSP), EPA 2023, EPA-HQ-OPP-2021 – 0756.
- Svingen T, Endocrine Disruptors in a New Era of Predictive Toxicology and Dealing With the “More is Different” Challenge, Front Toxicol 2022 Apr, 27;4:900479.
- Braeuning A, Balaguer P, Bourguet W, Carreras-Puigvert J, Feiertag K, Kamstra JH, Knapen D, Lichtenstein D, Marx-Stoelting P, Rietdijk J, Schubert K, Spjuth O, Stinckens E, Thedieck K, van den Boom R, Vergauwen L, von Bergen M, Wewer N and Zalko D, Development of new approach methods for the identification and characterization of endocrine metabolic disruptors — a PARC project, Front. Toxicol 2023, 5:1212509.


