This module introduces participants to the constantly evolving regulatory framework in France and Europe, which increasingly supports the replacement of animal use, while highlighting the scientific limitations and biases of traditional animal models. It clarifies the terminology related to New Approach Methodologies (NAMs) and presents the main types of non-animal tools available today, including in vitro systems and in silico models. The course also presents the main advantages of these approaches, explains the contexts in which they can be effectively implemented, and provides a brief overview of the international landscape, where global initiatives and regulatory bodies are accelerating the adoption of NAMs in research and testing.

Education Programme on NAMs
The Pro Anima Scientific Committee, through its scientific director Dr Lilas Courtot, offers comprehensive and ambitious training programmes on NAMs.
These educational programmes cover several complementary topics (general introduction, in vitro approaches, in silico approaches, regulatory aspects, research tools and practical implementation, etc.) and are designed to overcome a persistent structural barrier, well documented in PrecisionTox’s “Report on socio-technical barriers to the adoption of NAMs”: the vast majority of scientists, regulators, ethics committee members and even university lecturers have had very little exposure to new non-animal methods during their studies or careers, leading them to maintain the status quo and rely heavily on existing methods (usually based on mammals). Indeed, understanding of NAMs is often fragmented, outdated or limited to a conceptual overview rather than based on practical and applicable knowledge. Access to appropriate training is considered one of the most effective and urgent solutions to mitigate this barrier to wider adoption of NAMs. Pro Anima’s initiative directly addresses this need.
Training courses can be specially designed to meet the needs of different audiences: research project designers, animal facility staff, members of ethics committees or project evaluators for funding purposes.
You can contact us if you would like to organise NAM training at your institution or through your training organisation.
NAM training with e‑thiX Education
The Pro Anima Scientific Committee, through its scientific director Dr Lilas Courtot and in collaboration with e‑thiX Education, a certified training organisation in France, launched a comprehensive and ambitious training programme in 2025. The programme aims to equip researchers and scientific research professionals with knowledge of non-animal replacement methods and the confidence to transform their practices and adopt these innovative new tools.
Format
Each session lasts 3.5 hours (adjustable as needed), online or in person, in French or English, in order to be accessible to professionals throughout France and abroad. The teaching approach combines interactive lessons, guided exercises and assessment questionnaires, with each module ending with a certification. Participants also receive a comprehensive list of references and a resource guide, which allow them to explore the topics covered in greater depth and encourage them to continue their professional development beyond the training itself.
Target audience
The training courses are specially designed to meet the needs of different audiences: research project designers, animal facility staff, and members of ethics committees.
Content
The training programme currently consists of five complementary modules offering a coherent and progressive educational pathway.

1. Replacement : Introduction to Alternative Methods (non-animal)

2. Replacement : Searching for Alternative Methods (non-animal)
This module fills a critical gap: many researchers do not know where to begin when seeking non-animal solutions.This practical guidance helps participants in integrating the principle of replacement directly into the design and planning of scientific projects, ensuring that non-animal strategies are considered from the outset. It introduces key tools and resources for conducting systematic literature searches to identify alternative methods, including scientific databases, monitoring platforms, and structured approaches to staying updated on emerging techniques. Participants learn how to conduct an effective search for alternatives through appropriate keywords, search strategies, and evaluation criteria. The course also presents practical tools and methodological approaches to support the implementation of a new non-animal method in the laboratory, helping researchers transition from identification to concrete application in their daily scientific work.
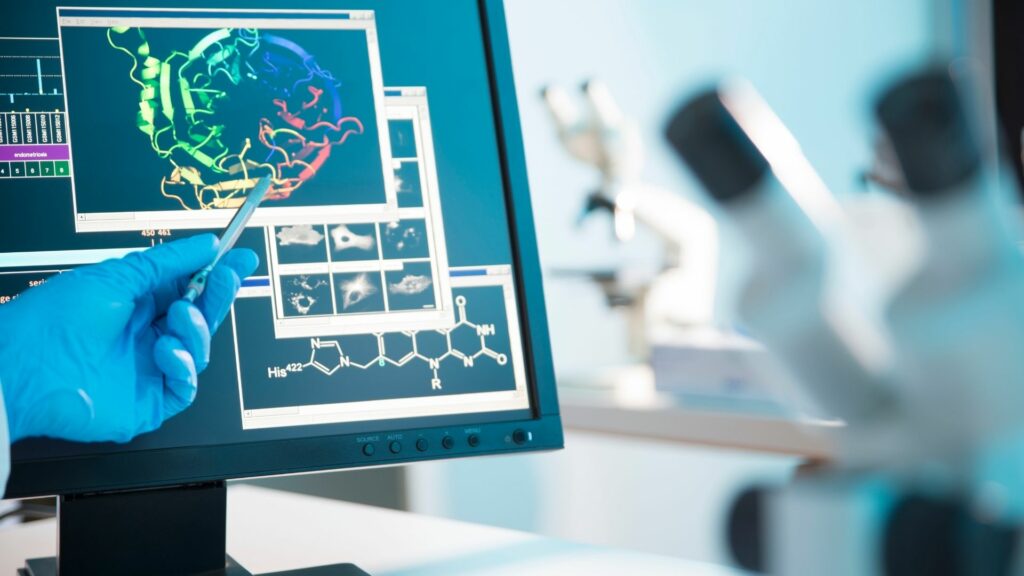
3. Replacement: Evaluating and Validating an Alternative Method (non-animal)
This module provides participants with a clear framework for evaluating and validating a non-animal method, beginning with pre-experimental assessment to determine technical feasibility, relevance, and scientific robustness before any practical implementation. It then examines the experimental approaches required to evaluate and validate a new method, focusing on reliability, reproducibility, performance criteria, and alignment with regulatory expectations. Post-experimental evaluation is also covered, enabling participants to interpret results critically and refine the method when necessary. The course offers an overview of scientific validation and regulatory validation pathways for the toxicological assessment of chemicals. It includes the roles of agencies such as the OECD and the complexity of their processes, supported by concrete case studies of successful acceptance of NAMs. By understanding these scientific and regulatory requirements, participants gain the confidence and expertise needed to implement a new alternative method effectively within their research environment.
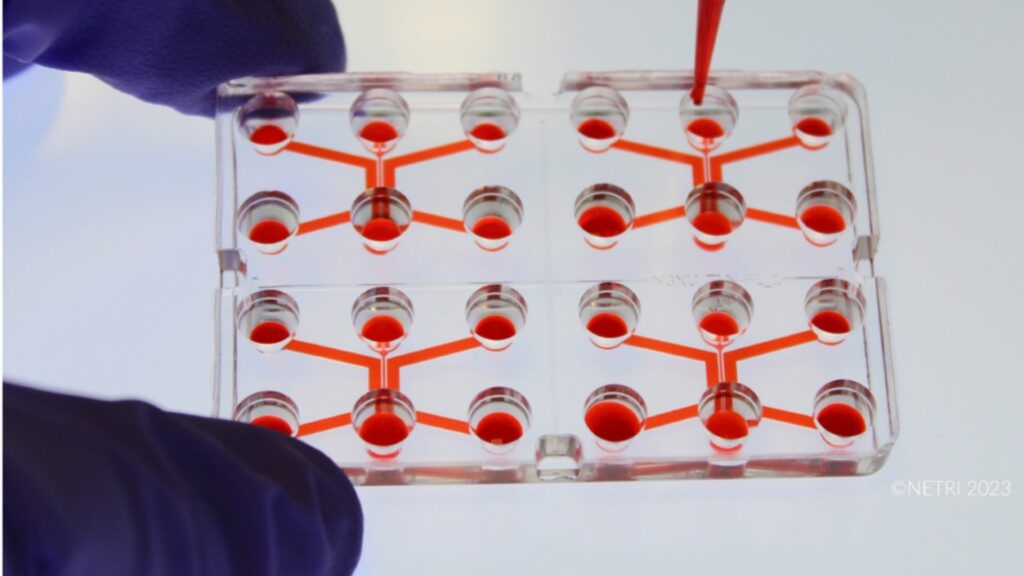
4. In Vitro Approaches: State of the Art in Organoïdes and Organ-On-Chips (OOC
This module offers an in-depth overview of the state of the art in advanced in vitro approaches, beginning with the history and definitions of 3D cell-based systems and explaining how organoids, stem-cell–derived models, and organ-on-chip (OOC) technologies have emerged as powerful human-relevant alternatives to traditional animal experimentation. Participants explore through key examples of applications the scientific advantages and broad application fields of organoids and OOC platforms—from toxicology and pharmacology to disease modelling and mechanistic studies—supported by concrete examples such as the Liver-chip for DILI (Drug-Induced Liver Injury), which illustrates their practical use and predictive value. The module also addresses the limitations and implementation barriers (qualification, standardisation, harmonisation) associated with these technologies, including technical, logistical, and regulatory challenges, and concludes by showing how in vitro systems can be combined with in silico approaches to create integrated, multi-scale strategies that further enhance the reliability and translational power of modern non-animal research.

5. In Silico Approaches: Artificial Intelligence, Simulation and Modelisation
This training module explores modern computational methods—from QSAR and PBPK models to machine learning, AI, and virtual twins—for predicting toxicity, modeling biological processes, and complementing in vitro approaches. It presents the historical foundations, advantages (accelerated research, reduced costs, integration of multi-scale data), and limitations (bias, technical barriers, validity of data and results, regulation) of these methods. Through concrete examples, participants discover how these tools are transforming research in toxicology and biology, while learning to evaluate their strengths and weaknesses. The module also addresses strategies for combining in silico and in vitro approaches, offering a balanced vision between innovation and pragmatism. It aims to provide the keys to integrating these approaches into scientific projects, optimizing their potential while anticipating their challenges.


