
Lucs / Valitox
An alternative to animal testing
Corrdinating by Pro Anima
And supported via the EthicScience Action Fund, by patrons including the French, Swiss and Belgian NGOs for the protection of animals and nature.
Research and Development : Anti-Oxidant Power – Laureate 2019 – 2020 EthicScience Prize
Denouncing animal iexperiments is one dimension of the problem.
The other side is proposing concrete solutions.
Wanting to test molecules intended for humans on animal species is an obsolete conception denying, on the one hand, the progress of science, and on the other hand, the fundamental barrier existing between each species.
It is also an aberration with regard to the conditions and the tests undergone by laboratory animals.
Valitox : a scientific alternative to animal experimentation
Following the European directive REACH, our program Valitox aims at better assessing the substances with which we are in contact, while avoiding cruel tests involving millions animals each year, especially the LD50 and LC50 tests.
Published in 2020, in the scientific journal Toxicology Reports (other link), our program has higher reliability than usual animal testing.
VALITOX in a few words
Valitox is a cell toxicology test that measures acute oral toxicity in humans without involving any animal testing.
Acute toxicity is defined by the adverse effects occurring after a short period of exposure to a substance (up to 24 hours).
Currently, acute oral toxicity is tested in animals according to OECD guidelines.
The Valitox cell test, innovative and based on the controlled production of oxidative stress by photo-induction of a molecule inside cells, makes it possible to assess the state of homeostasis (or “health”) of the cells, or their deterioration following exposure to toxic agents.
In other words, based on the technique of bio-fluorescence (working with / by the screening of light rays from human cells), Valitox makes it possible to detect the possible acute toxicity of a substance and thus measure the cellular deterioration in humans due to the effect of potential toxic agents.
Threatened cells react by reflecting light while healthy cells absorb it.
The Valitox test is being developed to eventually replace animal tests (LD50 and LC50) in toxicology ; as regards the pharmaceutical, cosmetic, food, agrochemical regulatory fields, etc.
Pro Anima has entrusted a leading laboratory, Anti-Oxidant Power based in Toulouse, with the development of the program, now registered under the names LUCS and Valitox.
To date, the Lucs / Valitox in vitro test has made it possible to test certain additives, dyes, preservatives and drugs withdrawn from the market and to demonstrate their toxicity or safety in vitro.
The innovative interest of the test has attracted the attention of some experts and the demonstration must be continued on a greater number of molecules.
VALITOX : in a few dates and publications
2006
Project initiation by Pro Anima
A first demonstration of the bio-fluorescence test is carried out in real time on human cells.
2007
Reproducibility phase under the scientific expertise of Prof. Jean-François Narbonne.
Objective : make the test reproducible on additional molecules.
2008
Recognition of the concept of bio-fluorescence and the advantage of the test’s predictability. The test reaches a predictivity of 82% against only 65% for the mouse tests and 61% for the rat tests.
(Animal tests remain the absolute reference although they have never had to undergo a validation procedure)
Publication of the results in 2009 in the journal ALTEX – edition of the Rome Congress (see below)
2009
Resolution of false negatives1 problems and presentation of the research at the 2009 Rome Congress on alternative methods.
Publication
J.F. Narbonne et al, DAP, a new fluorescent cell-based essay which predicts human acute toxicity to 82%, Altex (Alternatives to Animal Experimentation), ALTEX 26, Special Issue, 130, p.96, Thème 1 P09, 2009.
Voir l’ensemble du programme du Congrès
2012
Quand l’ADN détrône les souris, Sciences & Avenir hors-série, April 2012
2013 – 2015
Technological advancement with the elimination of the sample illumination step requiring the development of a special tool and that was limiting the dissemination of the test in laboratories.
2015
ECVAM2 pre-validation process, the European body for the validation of alternative methods to animal testing under the REACH program3
2016
Receipt of the ECVAM report following the pre-validation submission with the requirements to be pursued for the validation of the LUCS / VALITOX test
2017
Implementation of the demonstrations required by ECVAM according to a schedule extending until 2021
1st step : cellular mechanisms involved in the implementation of the alternative test.
Publication of results in the journal NATURE
Publication
S. DERICK, C. GIRONDE, P. PERIO. et al, LUCS (Light-Up Cell System), a universal high throughout assay for homeostasis evaluation in live cells, Nature, 22 décembre 2017, Sci Rep 7, 18069.
2019
2nd step : LUCS / VALITOX tested on bio-imprinted skins + new version of the VALITOX test, better suited to high-throughput test platforms, was proposed according to a simplified protocol.
2020
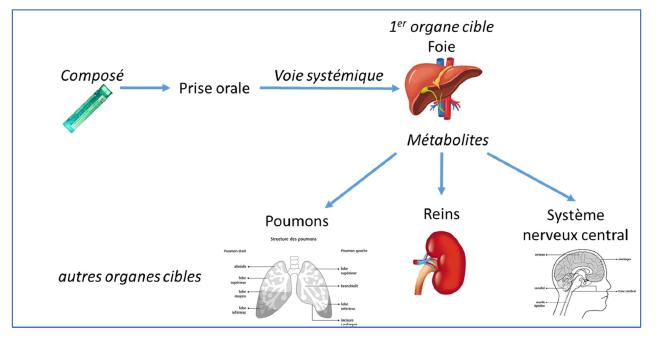
3rd step : For acute oral toxicology, the liver, due to its metabolic capacity, is a major target organ.
For this study demonstrating the effectiveness of VALITOX as a new alternative test method, the toxicity results obtained on human hepatocyte cells, exposed for 24 hours to 53 chemical compounds (drugs, pesticides, or industrial products), were compared with bibliographic data on in vivo toxicity for these same substances.
LUCS / VALITOX cell test makes it possible to predict the toxic effects of a substance in humans (on liver cells) with a predictive rate of 69% against a rate between 50 and 57% for tests on animals.
Publication
C. FURGER et al, Use of LUCS (Light-Up Cell System) / VALITOX as an alternative live cell method to predict human acute oral toxicity, Toxicology Reports, 2020, Vol. 7, p403-412.
Lire la synthèse du rapport Valitox 2020
Lien vers document pdf synthèse valitox dans valise
2021
4th step : In order to provide a complete scheme of human toxicity, the evaluation of toxicity on secondary target organs : kidneys, lungs and central nervous system is planned.
2022
In March 2022, Christophe Furger, director of French Lab AOP and scientific director of the LUCS/Valitox cell test, was invited by ARET (Association for Research in Toxicology) as part of the association’s webinar program to present the cell test.
The lecture, entitled “LUCS (Light Up Cell System). A new alternative method to animal tests for the evaluation of systemic toxicity and other regulatory applications” was the occasion to present and review :
- The different advantages of the Valitox approach (related to its robustness, its cost, its procedure…)
- The multiplex analysis (simultaneous determination of a large number of biological molecules in a single test)
- Possible applications of LUCS / Valitox technology on industrial tools
- Validation of the test on skin models
- The validated alternative methods
- The results of LUCS / Valitox studies conducted in 2021
- The submission to ECVAM in 2021 and the opening on future perspectives, especially with organs on chip and organotypic cultures.
See more details with the lecture slides.



